Background
The standard of care for loco-regional RCC is nephrectomy, but many patients (pts) experience recurrence. IMmotion010, a Phase III, multicentre, randomised, pbo-controlled, double-blinded trial, evaluated atezo (anti-PD-L1) monotherapy as adjuvant therapy in pts with RCC and increased risk of recurrence after resection.
Methods
Key eligibility criteria included pts with RCC with a clear-cell or sarcomatoid component and who had increased risk of recurrence (T2 Grade [Gr] 4, T3a Gr 3/4, T3b/c or T4 any Gr, TxN+ any Gr or M1 resected with no evidence of disease). Pts were randomised 1:1 to atezo 1200 mg IV q3w or pbo IV q3w for 16 cycles or 1 year. The primary endpoint was investigator (INV)-assessed disease-free survival (DFS). Secondary endpoints included OS and independent review facility (IRF)-assessed DFS in the ITT population, and INV-DFS and IRF-DFS in pts with PD-L1 immune cell expression ≥1% (VENTANA SP142 IHC assay).
Results
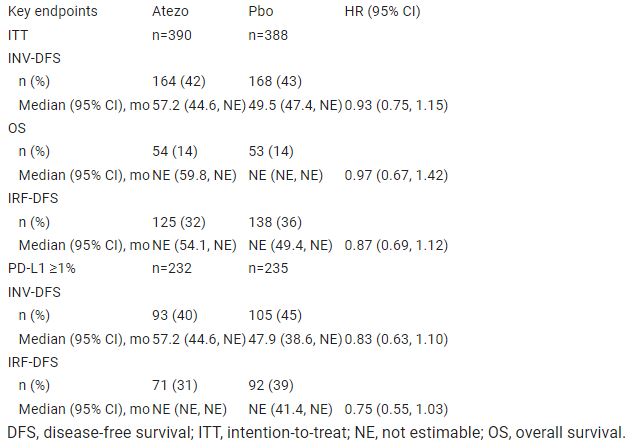
From 3 Jan 2017 to 15 Feb 2019, 778 pts were randomised to atezo (n=390) or pbo (n=388). At primary analysis data cutoff (3 May 2022), median follow-up was 44.7 mo and minimum follow-up was 38.6 mo. No pts remain on study treatment. Baseline characteristics were generally balanced between arms. Median INV-DFS was 57.2 mo (95% CI: 44.6, NE) for atezo and 49.5 mo (47.4, NE) for pbo (HR: 0.93; 95% CI: 0.75, 1.15; P=0.495). Key secondary endpoints are in the Table. In the safety population, Gr 3/4 adverse events (AEs) occurred in 27% (106/390) and 21% (81/383) of pts receiving atezo or pbo, respectively; Gr 5 AEs occurred in <1% (1/390) and <1% (3/383), none related to treatment.
Conclusions
Atezo as adjuvant therapy after resection for pts with RCC with increased risk of recurrence did not improve clinical outcomes vs pbo in the ITT population but had a manageable safety profile. Subgroup data will be presented. Table Table: 000LBA66
Clinical trial identification
NCT03024996.