Background
Ipilimumab (IPI) plus nivolumab (N) is a standard first-line treatment for patients (pts) with intermediate and poor-risk advanced renal cell carcinoma (aRCC). Grade 3/4 (G3/4) treatment-related adverse events (trAE) are relatively common during the initial combination period. The aim of this randomized phase II trial was to determine whether modified scheduling of IPI, in combination with N, is associated with improved tolerability, whilst maintaining treatment efficacy in line with previous comparative studies with sunitinib.
Methods
Pts with untreated clear cell aRCC were randomized 1:2 to receive 4 doses of IPI 1mg/kg Q3W (conventional IPI) or Q12W (modified IPI), in combination with N (3mg/kg), until disease progression or unacceptable toxicity. The primary endpoint was the proportion of pts with a G3/4 trAE within 12 months of initiating treatment (from those who received at least one dose of therapy (modified intention-to-treat)). Secondary endpoints included progression-free survival (PFS) at 12 months and objective response rate (ORR).
Results
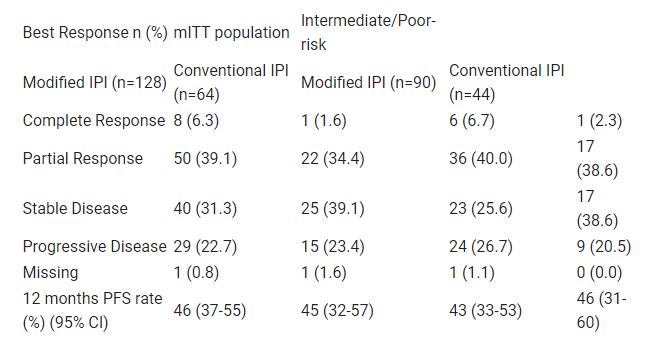
192 pts (69.8% intermediate/poor-risk) received at least one dose of study drug. G3/4 trAE were significantly lower amongst pts receiving modified IPI compared to conventional IPI (32.8% v 53.1%; OR 0.43 [90% CI: 0.25, 0.72]; p=0.0075). Efficacy endpoints are given in the table and were similar between treatment arms and pre-specified IMDC risk subgroups.
Conclusions
Giving IPI 12-weekly, instead of 3-weekly, in combination with N, was associated with a clinically significant reduction in rates of G3/4 trAE. Outcome data suggested there was no clear reduction in ORR or PFS with the modified schedule and is in line with previous comparative studies with sunitinib (Table). Table: LBA29
Clinical trial identification
EudraCT 2017-001476-33.
Editorial acknowledgement
Legal entity responsible for the study
University of Leeds/Leeds Teaching Hospitals NHS Trust.
Funding
Bristol Myers Squibb.
Disclosure
N.S. Vasudev: Financial Interests, Personal, Advisory Board: BMS; Non-Financial Interests, Institutional, Research Grant: BMS; Financial Interests, Personal, Speaker’s Bureau: BMS; Financial Interests, Personal, Invited Speaker: EUSA Pharma; Financial Interests, Personal, Advisory Board: EUSA Pharma; Financial Interests, Personal, Invited Speaker: Ipsen; Financial Interests, Personal, Invited Speaker: Pfizer; Financial Interests, Personal, Advisory Board: Merck Serono; Financial Interests, Personal, Advisory Board: 4D Pharma. L. Pickering: Financial Interests, Personal, Invited Speaker: BMS; Financial Interests, Personal, Advisory Board: BMS; Financial Interests, Personal, Invited Speaker: Eisai; Financial Interests, Personal, Advisory Board: MSD; Financial Interests, Personal, Advisory Board: Novartis; Financial Interests, Personal, Advisory Board: Pfizer; Financial Interests, Personal, Invited Speaker: Pfizer; Financial Interests, Institutional, Research Grant: NIHR. T.S. Waddell: Financial Interests, Personal, Invited Speaker: Pfizer; Financial Interests, Personal, Invited Speaker: Ipsen; Financial Interests, Personal, Invited Speaker: BMS; Financial Interests, Personal, Invited Speaker: EUSA Pharma; Financial Interests, Personal, Advisory Board: Roche; Financial Interests, Personal, Advisory Board: Pfizer; Financial Interests, Personal, Advisory Board: Ipsen; Financial Interests, Personal, Advisory Board: BMS; Financial Interests, Personal, Advisory Board: MSD; Financial Interests, Personal, Advisory Board: Eisai; Financial Interests, Institutional, Research Grant: BMS; Financial Interests, Institutional, Research Grant: Pfizer; Financial Interests, Institutional, Research Grant: Ipsen; Financial Interests, Institutional, Research Grant: Roche; Financial Interests, Institutional, Research Grant: Eisai. K. Fife: Financial Interests, Personal, Advisory Board: BMS. R. Griffiths: Financial Interests, Personal, Invited Speaker: BMS; Financial Interests, Personal, Invited Speaker: Ipsen; Financial Interests, Personal, Invited Speaker: MSD; Financial Interests, Personal, Invited Speaker: Eisai; Financial Interests, Personal, Advisory Board: BMS; Financial Interests, Personal, Advisory Board: Eisai. A. Sharma: Financial Interests, Personal, Advisory Board: Pfizer; Financial Interests, Personal, Advisory Board: BMS; Financial Interests, Personal, Advisory Board: MSD; Financial Interests, Personal, Advisory Board: Merck; Financial Interests, Personal, Invited Speaker: Ipsen; Financial Interests, Personal, Invited Speaker: Eisai. G. Velikova: Financial Interests, Personal, Advisory Board: Roche; Financial Interests, Personal, Advisory Board: Eisai; Financial Interests, Personal, Advisory Board: Novartis; Financial Interests, Personal, Advisory Board: Seattle Geneics; Financial Interests, Institutional, Research Grant: Breast Cancer Now; Financial Interests, Institutional, Research Grant: EORTC; Financial Interests, Institutional, Research Grant: Yorkshire Cancer Research; Financial Interests, Institutional, Research Grant: Pfizer Inc; Financial Interests, Institutional, Research Grant: IQVIA. A. Maraveyas: Financial Interests, Personal, Advisory Board: BMS; Financial Interests, Personal, Advisory Board: Pfizer; Financial Interests, Personal, Advisory Board: Bayer; Financial Interests, Personal, Advisory Board: Leo; Financial Interests, Personal, Invited Speaker: Bayer; Financial Interests, Personal, Invited Speaker: Pfizer; Financial Interests, Personal, Invited Speaker: Leo; Financial Interests, Institutional, Research Grant: BMS; Financial Interests, Institutional, Research Grant: Boehringer Ingelheim; Financial Interests, Institutional, Research Grant: Bayer; Financial Interests, Institutional, Research Grant: Leo. J.E. Brown: Financial Interests, Personal, Advisory Board: Novartis; Financial Interests, Personal, Advisory Board: Ipsen; Financial Interests, Personal, Advisory Board: MSD; Financial Interests, Personal, Advisory Board: Daiichi Sankyo; Financial Interests, Personal, Advisory Board: Sandoz; Financial Interests, Institutional, Research Grant: Amgen; Financial Interests, Institutional, Research Grant: Bayer. B. Venugopal: Financial Interests, Personal, Invited Speaker: BMS; Financial Interests, Personal, Advisory Board: BMS; Financial Interests, Personal, Advisory Board: Eisai; Financial Interests, Personal, Advisory Board: EUSA pharma; Financial Interests, Personal, Invited Speaker: Ipsen; Financial Interests, Personal, Invited Speaker: MSD; Financial Interests, Personal, Advisory Board: MSD; Financial Interests, Personal, Invited Speaker: Merck Serono; Financial Interests, Personal, Invited Speaker: Pfizer; Non-Financial Interests, Institutional, Principal Investigator: Ipsen; Non-Financial Interests, Institutional, Principal Investigator: Calithera; Non-Financial Interests, Institutional, Principal Investigator: Exelixis. P. Patel: Financial Interests, Personal, Invited Speaker: BMS. S. Symeonides: Financial Interests, Institutional, Advisory Board: BMS; Financial Interests, Institutional, Advisory Board: Ellipses; Financial Interests, Institutional, Advisory Board: EUSA pharma; Financial Interests, Institutional, Advisory Board: Medannex; Financial Interests, Institutional, Advisory Board: Merck Serono; Financial Interests, Institutional, Advisory Board: MSD; Financial Interests, Institutional, Advisory Board: Pfizer; Financial Interests, Institutional, Advisory Board: Vaccitech; Non-Financial Interests, Institutional, Principal Investigator: BioLineRx; Financial Interests, Institutional, Research Grant: MSD; Financial Interests, Institutional, Research Grant: Roche; Financial Interests, Institutional, Research Grant: Verastem. P.D. Nathan: Financial Interests, Invited Speaker: Pleas see ESMO DOI. T.B. Powles: Financial Interests, Personal, Advisory Board: Merck Serono; Financial Interests, Personal, Advisory Board: MSD; Financial Interests, Personal, Advisory Board: BMS; Financial Interests, Personal, Advisory Board: Roche; Financial Interests, Personal, Advisory Board: AstraZeneca; Financial Interests, Personal, Advisory Board: Astellas; Financial Interests, Personal, Advisory Board: Novartis; Financial Interests, Personal, Advisory Board: J&J; Financial Interests, Personal, Advisory Board: Seattle Genetics; Financial Interests, Personal, Advisory Board: Pfizer; Financial Interests, Personal, Advisory Board: Exelexis; Financial Interests, Personal, Advisory Board: Eisai; Financial Interests, Institutional, Research Grant: BMS; Financial Interests, Institutional, Research Grant: Merck Serono; Financial Interests, Institutional, Research Grant: MSD; Financial Interests, Institutional, Research Grant: Roche; Financial Interests, Institutional, Research Grant: AstraZeneca; Financial Interests, Institutional, Research Grant: Astellas; Financial Interests, Institutional, Research Grant: Novartis; Financial Interests, Institutional, Research Grant: J&J; Financial Interests, Institutional, Research Grant: Seattle Genetics; Financial Interests, Institutional, Research Grant: Pfizer; Financial Interests, Institutional, Research Grant: Exelexis; Financial Interests, Institutional, Research Grant: Eisai. All other authors have declared no conflicts of interest.