Research Funding
Bristol Myers Squibb.
Background
In the phase III, open-label CheckMate 9ER trial (NCT03141177), patients with aRCC were randomized 1:1 (stratified by International Metastatic Renal Cell Carcinoma Database Consortium risk score, tumor programmed death ligand 1 expression, geographic region) to nivolumab 240 mg IV Q2W + cabozantinib 40 mg PO QD (N+C; n = 323) or sunitinib (S) 50 mg PO (4 weeks of 6-week cycles; n = 328) for first-line treatment until disease progression or unacceptable toxicity (max N treatment, 2 years). N+C met primary and secondary efficacy endpoints by significantly improving progression-free survival, overall survival, and objective response rate versus S in aRCC patients with a clear cell component. Here, we present in-depth health-related quality of life (HRQoL) patient-reported outcome (PRO) results, including overall between-group comparisons of treatment groups and time to confirmed deterioration (TCD).
Methods
PROs in all randomized patients were an exploratory endpoint assessed using the Functional Assessment of Cancer Therapy Kidney Symptom Index-19 (FKSI-19) and EQ-5D-3L instruments. PRO assessments at baseline, common on-treatment scheduled visits, and common follow-up visits for both arms were analyzed. Changes from baseline were assessed using mixed-model repeated measures (MMRM), adjusting for baseline scores and stratification factors. TCD was calculated from Kaplan–Meier estimates and Cox proportional hazards models.
Results
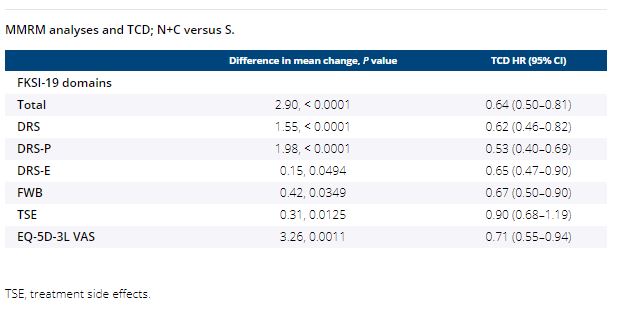
Median follow-up for overall survival was 18.1 months. PRO completion rates were > 90% at baseline, and ≥ 80% at all on-treatment assessments (≥ 10 patients) through week 91 in both arms. The overall least squares mean difference in change from baseline favored N+C over S in FKSI-19 (all domains) and in EQ-5D-3L. Patients treated with N+C experienced less treatment burden, with decreased risk of confirmed deterioration across most measurements versus S, including FKSI-19 total, disease-related symptoms (DRS), DRS-physical (DRS-P), DRS-emotional (DRS-E), functional well-being (FWB), and EQ-5D-3L visual analog scale (VAS) scores (Table).
Conclusions
Patients reported statistically significant HRQoL benefits with N+C versus S. Treatment with N+C significantly reduced the risk of deterioration in HRQoL scores, including in disease-related symptoms of kidney cancer. These results suggest that the superior efficacy of N+C over S comes with the additional benefit of improved HRQoL. Clinical trial information: NCT03141177