Background
The randomized, open-label, phase 3 KEYNOTE-426 study (NCT02853331) met its primary and key secondary end points of improved OS, PFS, and ORR with pembro + axi versus sunitinib as first-line treatment for patients with advanced ccRCC. Extended follow-up (42.8-mo median follow-up) continued to show the superior efficacy of pembro + axi versus sunitinib in this patient population. We describe the results of PFS2 for all randomly assigned patients and across IMDC risk categories.
Methods
Treatment-naive patients with advanced ccRCC, Karnofsky Performance Status Scale score ≥70% and measurable disease per RECIST v1.1 were randomly assigned 1:1 to receive pembro 200 mg IV every 3 weeks for up to 35 doses (̃2 y) + axi 5 mg orally twice daily or sunitinib 50 mg orally once daily on a 4-wk on/2-wk off schedule. The end point of this exploratory analysis was PFS2, defined as time from randomization to progression after first subsequent therapy or any-cause death. The Kaplan-Meier method was used to estimate PFS2 and hazard ratios were estimated using a Cox regression model.
Results
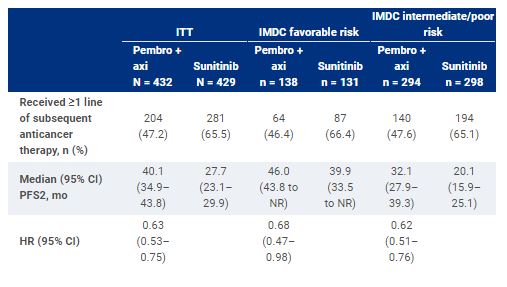
Of 861 patients, 432 were assigned to receive pembro + axi; 429, to sunitinib. Median time from randomization to the database cutoff date (January 11, 2021) was 42.8 mo (range, 35.6-50.6). Overall, 47.2% of patients (204/432) in the pembro + axi arm and 65.5% of patients (281/429) in the sunitinib arm received ≥1 line of subsequent anticancer therapy. For patients who received subsequent therapy, anti–PD-1/PD-L1 agents were the first subsequent treatment for 11.3% of patients (23/204) in the pembro + axi arm and 54.8% of patients (154/281) in the sunitinib arm. In the pembro + axi arm, 82.8% of patients (169/204) received a VEGF/VEGFR inhibitor as first subsequent therapy, as did 43.4% (122/281) in the sunitinib arm. PFS2 results are displayed in the Table.
Conclusions
In this exploratory analysis, PFS2 was longer for patients randomized to pembro + axi compared to sunitinib. Results were consistent across IMDC risk groups. These data support use of pembro + axi for the first-line treatment of patients with advanced ccRCC. Clinical trial information: NCT02853331.