Research Funding
Eisai Inc., Woodcliff Lake, NJ, USA; and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Background
In pts with advanced RCC, second-line treatment with LEN + EVE prolonged progression-free survival (PFS) compared with EVE alone. LEN + PEMBRO, also showed preliminary efficacious evidence in a phase 1/2 RCC study. Here, we describe the investigational study results of first-line LEN + PEMBRO or LEN + EVE versus SUN in pts with advanced RCC.
Methods
Pts were randomized (1:1:1) to receive LEN 20 mg orally once daily + PEMBRO 200 mg IV every 3 weeks (wks); or LEN 18 mg + EVE 5 mg orally once daily; or SUN 50 mg orally once daily (4 wks on/2 wks off). Eligible pts had advanced RCC with no prior systemic therapy. Randomization was stratified by geographic region and MSKCC prognostic group. The primary endpoint was PFS by Independent Review Committee per RECIST v1.1. Secondary endpoints included overall survival (OS), objective response rate (ORR) and safety. A sequential approach was used to test PFS first, then OS and ORR. PFS and OS were compared across arms by a stratified log-rank test; hazard ratios (HRs) were estimated by a stratified Cox regression model.
Results
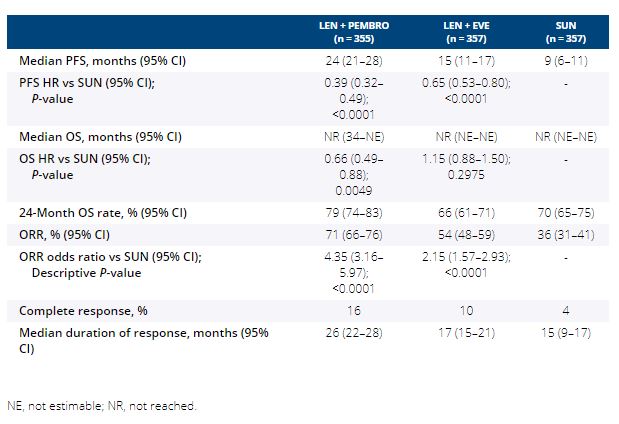
1069 pts were randomized (Table). After a median follow-up of 27 months (data cutoff August 28, 2020), PFS was significantly improved with LEN + PEMBRO (median 24 months [mos]) vs SUN (median 9 mos; HR 0.39, 95% CI 0.32–0.49) and LEN + EVE (median 15 mos) vs SUN (HR 0.65, 95% CI 0.53–0.80). OS was significantly longer with LEN + PEMBRO vs SUN (HR 0.66, 95% CI 0.49–0.88), whereas OS with LEN + EVE vs SUN was not statistically different (HR 1.15, 95% CI 0.88–1.50). ORR was significantly greater with LEN + PEMBRO (ORR 71%; complete response [CR] 16%) vs SUN (ORR 36%; CR 4%; odds ratio 4.35, 95% CI 3.16–5.97) and LEN + EVE (ORR 54%; CR 10%) vs SUN (odds ratio 2.15, 95% CI 1.57–2.93). Grade ≥3 treatment-related adverse events occurred in 72% of pts in the LEN + PEMBRO arm and 73% of pts in the LEN + EVE arm compared with 59% of pts in the SUN arm.
Conclusions
LEN + PEMBRO demonstrated significant improvements in PFS, OS and ORR vs SUN. LEN + EVE demonstrated significant improvements in PFS and ORR vs SUN. Safety was manageable and consistent with the known single-agent profiles. Clinical trial information: NCT02811861.