Research Funding
Pharmaceutical/Biotech Company
Pfizer
Background
In renal cell carcinoma (RCC), partial nephrectomy (PN) is indicated for patients with solitary kidney, chronic kidney disease, or bilateral tumors. A subset of these patients, however, may have large and complex renal masses not initially suitable for PN. Neoadjuvant Tyrosine Kinase Inhibitor therapy has shown promising results in cytoreducing renal tumors and may permit PN in circumstances not otherwise feasible.
Methods
This was a single arm phase II clinical trial of neoadjuvant axitinib in patients with complex (RENAL nephrometry score 10-12 and cT1b-cT3M0) biopsy-proven clear cell RCC with strong indications for partial nephrectomy (PN), and in whom radical nephrectomy may result in dialysis dependence. Axitinib 5 mg was administered orally twice daily for 8 weeks prior to surgery. Primary outcome was reduction in longest tumor diameter; secondary outcomes included tumor response (RECIST), change in RENAL score, feasibility of PN, change in estimated glomerular filtration rate (DeGFR), and post-surgical complications.
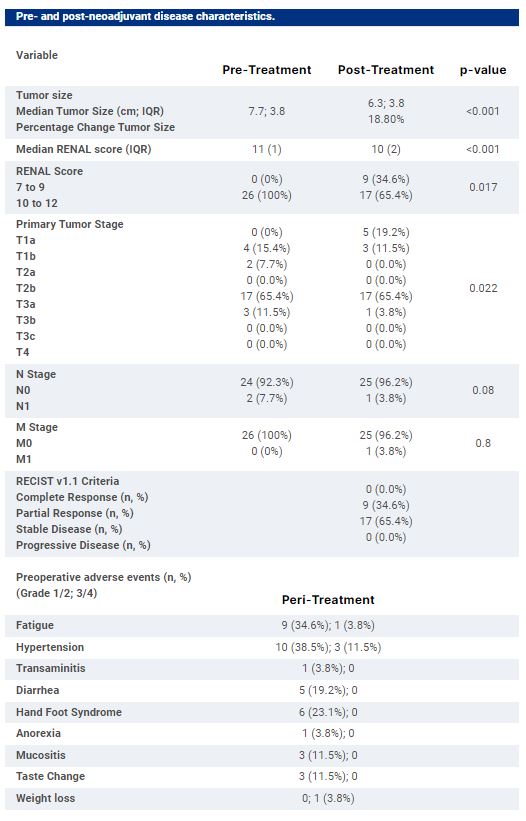
Results
26 patients were enrolled. 19 (73.1%) patients had ≥ clinical T3a staged tumors. Post therapy, 17 (65.4%) patients had ≥T3a staged tumors. Axitinib resulted in reductions in tumor size (7.7 vs. 6.3 cm, p<0.001) and RENAL score (11 vs. 10, p <0.001); 9 (34.6%) had partial response, and 17 (65.4%) stable disease by RECIST criteria. PN was successfully performed in 19 (73.1%); 24 (96.8%) achieved negative margins. Six (23.1%) had Clavien III-IV post-surgical complications. Median percentage DeGFR was 14.7%; one (3.8%) patient who had a radical nephrectomy had long-term dialysis dependence.Conclusions:Neoadjuvant axitnib resulted in significant reductions in tumor size and complexity, enabling PN in a cohort of complex renal masses, and with acceptable safety and functional preservation. Clinical trial information: NCT03438708.