Background
The primary analysis of health-related quality of life (HRQoL) from CheckMate 214 have been published (Cella et al. Lancet Oncol. 2019). Nivolumab + ipilimumab (N+I) led to superior overall survival (OS) (HR: 0.63; p < 0.001) and more favorable HRQoL than sunitinib (S) as 1st-line treatment for intermediate/poor (I/P)-risk patients (pts) with aRCC. We report herein the HRQoL analyses from the 30-month follow-up.
Methods
Pts were randomized 1:1 to receive N 3 mg/kg + I 1 mg/kg every 3 wk for 4 doses then N 3 mg/kg every 2 wk, or S 50 mg/d orally for 4 wk (6-wk cycle). HRQoL was assessed on day 1 of wks 1 and 4 of the first 2 cycles, on day 1 of wks 1 and 5 of the next 2 cycles and on day 1 of wk 1 of subsequent cycles. An exploratory HRQoL analysis was conducted using the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI-19), Functional Assessment of Cancer Therapy-General (FACT-G) and EQ-5D instruments. The analyses included mixed-model repeated measures (MMRM) for change from baseline (BL) at 145 wks (while on-treatment), and time to deterioration (TTD).
Results
Table:
951P MMRM analysis (treatment differences at week 145) and time to deterioration for FKSI-19
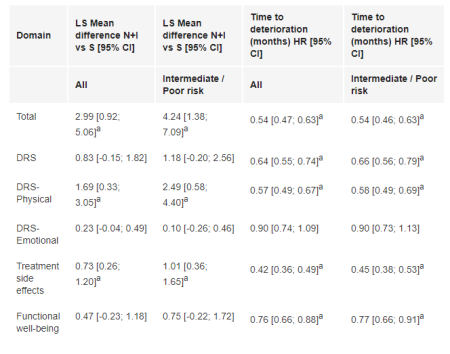
1096 pts were randomized to N+I (I/P risk: 425; favorable [F] risk: 125) and S (I/P risk: 422; F risk: 124). HRQoL assessment completion rates were >78% in the first 145 wks. In the total and I/P-risk populations, N+I pts report improved FKSI-19 total scores over time to wk 145 while decrease is observed with S. At 145 wks (Table), the difference in change from BL between arms for FKSI-19 total, disease-related symptoms-physical and treatment side effects scores significantly benefited N+I vs S. TTD was statistically significantly longer with N+I for most domains in both populations. Similar results were observed for FACT-G and EQ-5D change from BL and TTD.
Conclusions
N+I significantly improved OS vs S without worsening HRQoL. N+I sustained long-term good HRQoL and significantly delayed TTD in both the total and I/P-risk populations.