Background
Nivolumab-ipilimumab (NI) and sunitinib/pazopanib (TKI) are indicated in m-ccRCC pts with IMDC intermediate/poor and good risk groups, respectively. Based on unsupervised analysis of genes expressed in m-ccRCC, we identified 4 groups (ccrcc1-4) with immune and angiogenic high/low features which could allow to better identify responders to either N, NI or TKI. We here report final analysis results of the BIONIKK trial.
Methods
BIONIKK is an open-label, French multicenter randomized phase 2 trial evaluating N vs. NI vs. TKI in upfront m-ccRCC according to ccrcc1-4 (35-gene signature, CITsig). ccrcc1,4 and ccrcc2,3 pts were randomized to receive N vs. NI and NI vs. TKI respectively. Primary endpoint (PE): objective response rate (ORR, RECIST1.1) per treatment and group. Secondary endpoints: progression-free survival (PFS), overall survival (OS) and tolerability. 150 pts were expected in target cohort (TC). An additional cohort was included to assess inter-platform variability.
Results
From 06/2017 to 07/2019: screened=308, randomized=202 (ALL), evaluable for PE=187 with TC=154. No correlation between ccrcc1-4 and IMDC risk groups (p=0.14). ORR (TC): N=30%, NI=44%, TKI=50%, with differences according to ccrcc1-4 (table1). ORR for NI were comparable across all groups. In ccrcc1 ORR for N was half that of NI while both were comparable in ccrcc4. In ccrcc2, ORR for TKI was as high as for NI. After a median (m) FU of 16 months (mo), mPFS (TC, mo): N=4.9, NI=10.4, TKI=NR, with again observed differences according to ccrcc1-4. OS data are not mature (events:16%). No new safety signal emerged compared to published data. CITsig in frozen and FFPE samples will be presented at the meeting.
Table: LBA25
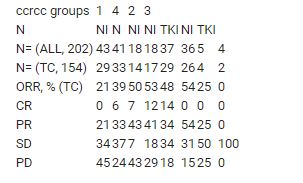
We demonstrate for the 1st time in m-ccRCC pts that gene expression signatures may enable to enrich response rates. An extensive translational program is planned to identify new biomarkers.