Research Funding:
Bob Parker Fund for Kidney Cancer Research, Pharmaceutical/Biotech Company.
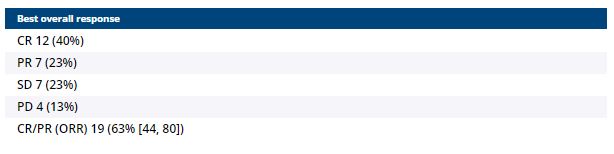
Background:Pembrolizumab monotherapy, whilst not standard of care, has demonstrated efficacy in clear cell renal cell carcinoma (ccRCC). The first-line KEYNOTE-427 study demonstrated an overall response rate (ORR) of 34%, and a median progression-free survival (PFS) of 7.1 mo (McDermott D et al. J Clin Oncol 2020; 38:S15; 5069-5069). Stereotactic ablative body radiotherapy (SABR) is an option for oligometastatic ccRCC, but patients often develop distant progression or relapse within irradiated sites. The RAPPORT study (NCT02855203) was a multi-institutional single arm, phase I/II study evaluating safety and efficacy of SABR and pembrolizumab.Methods:Patients with up to 2 lines of prior systemic therapy with 1-5 oligometastases from ccRCC were eligible. A single fraction of 20Gy SABR to all metastatic sites was given (or 10 fractions of 3 Gy of conventional radiotherapy [CRT] if SABR was not feasible), followed by pembrolizumab 200mg administered Q3W for 8 cycles. The primary objective was safety (CTCAEv4.03), with secondary key objectives of efficacy (RECIST1.1) by disease control rate (DCR), defined as complete response (CR), partial response (PR) or stable disease for at least 6 months, ORR, PFS and overall survival (OS).Results:Thirty patients were enrolled and received protocol treatment. The median follow-up was was 2.3 years. The median age was 62 (range 47-80) years, 23 patients (77%) were male. Twenty-three patients (77%) were treatment naïve, 1 patient (3%) had a prior interleukin-2 therapy and 6 patients (20%) had a prior tyrosine kinase inhibitor. Nine patients (30%) had prior metastasectomy. Eighty-three oligometastases were treated (median of 3 per patient), of which 64 (77%) received SABR, and 19 (23%) received CRT. There were 8 adrenal, 11 bone, 43 lung, 12 lymph node and 9 soft tissue metastases irradiated. Four patients (13% [95%CI: 4-31%]) had one or more grade 3 treatment-related AE: Pneumonitis (n=2), dyspnoea (n=1) and elevated ALP/ALT (n=1). There were no grade 4 or 5 AEs. All eight cycles of pembrolizumab were completed by 24 (80%) patients. DCR was 83% (95%CI: 65-94%). ORRs are tabulated below. Median PFS was 15.6 mo. Estimated 1 and 2-year OS was 90% (95%CI: 72-97%) and 74% (95%CI: 53-87%), respectively, while PFS was 60% (95%CI: 40-75%) and 45% (95%CI: 27-62%), respectively. Freedom from local progression at 2-years was 92% (95%CI: 80-97%).Conclusions:The combination of SABR and pembrolizumab in oligometastatic renal cell carcinoma is well tolerated with excellent local control. Durable responses and encouraging PFS were observed with this approach, which warrants further investigation. Clinical trial information: NCT02855203.