Background
In the phase III KEYNOTE-426 study, pembrolizumab + axitinib showed significant improvement in OS, PFS, and ORR vs sunitinib in patients with RCC. This analysis assessed subsequent treatment in patients enrolled in KEYNOTE-426.
Methods
Treatment-naive patients with clear cell RCC, KPS score ≥70%, and measurable disease (RECIST v1.1) were randomly assigned 1:1 to receive pembrolizumab 200 mg IV every 3 weeks for up to 35 doses + axitinib 5 mg orally twice daily or sunitinib 50 mg once daily (4 weeks on/2 weeks off) until progression, toxicity, or withdrawal. Type of and time to subsequent therapy were assessed.
Results
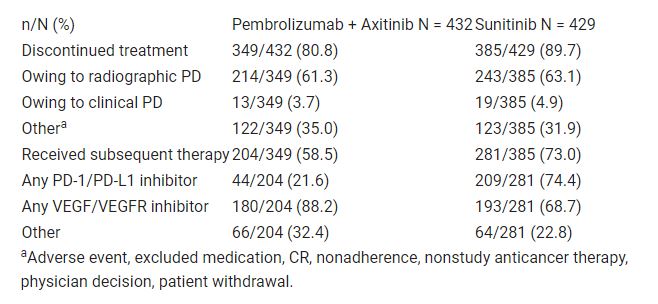
Of patients in the pembrolizumab + axitinib arm and in the sunitinib arm, 81.4% (349/432) and 90.6% of patients (385/429), respectively, discontinued treatment; radiologic or clinical PD was the most common reason for discontinuation in both (pembrolizumab + axitinib: 65.0% [227/349]; sunitinib: 68.1% [262/385]). Of patients who discontinued, 58.5% of patients (204/349) in the pembrolizumab + axitinib arm and 73.0% (281/385) in the sunitinib arm received subsequent therapy (Table). Although a similar proportion of patients in both arms received subsequent therapy with a VEGF/VEGFR inhibitor (pembrolizumab + axitinib: 88.2% [180/204]; sunitinib: 68.7% [193/281]), a greater proportion of patients in the sunitinib arm (74.4% [209/281]) received subsequent PD-1/PD-L1 inhibitor therapy than in the pembrolizumab + axitinib arm (21.6% [44/204]). Of patients in the pembrolizumab + axitinib arm and the sunitinib arm, 32.4% (66/204) and 22.8% (64/281), respectively, received other therapies.
Conclusions
The superior efficacy of pembrolizumab + axitinib compared with sunitinib is observed despite the increased use of subsequent therapy in the sunitinib arm. These data continue to support the use of first-line pembrolizumab + axitinib in patients with RCC. Table: 669P
Clinical trial identification
NCT02853331; August 2, 2016.
Editorial acknowledgement
Medical writing and/or editorial assistance was provided by Matthew Grzywacz, PhD, of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Legal entity responsible for the study
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Funding
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Disclosure
R. Gafanov: Financial Interests, Institutional, Other, Honoraria: Janssen, MSD, Bayer, AstraZeneca; Financial Interests, Personal, Advisory Role: Astellas, Janssen, Sanofi, Pfizer, BMS, MSD, Bayer, Eisai, Ipsen, Pierre Fabre, AstraZeneca; Financial Interests, Personal, Speaker’s Bureau: Astellas, Janssen, Sanofi, Pfizer, BMS, MSD, Bayer, Eisai, Ipsen, Pierre Fabre, AstraZeneca; Financial Interests, Institutional, Research Grant: Janssen, MSD, Bayer, AstraZeneca. T.B. Powles: Financial Interests, Personal, Advisory Role: Bristol Myers Squibb, Merck, AstraZeneca, Ipsen, Pfizer, Novartis, Incyte, Seattle Genetics, Roche, Exelixis, MSD, Merck Serono, Astellas Pharma, Johnson & Johnson, Eisai; Financial Interests, Personal, Other, Travel Expenses: Pfizer, MSD, AstraZeneca, Roche, Ipsen; Financial Interests, Personal, Other, Honoraria: AstraZeneca, Bristol Myers Squibb, Exelixis, Incyte, Ipsen, Merck, MSD, Novrtis, Pfizer, Seattle Genetics, Merck Serono, Astellas Pharma, Johnson & Johnson, Eisai, Roche; Financial Interests, Personal, Research Grant: AstraZeneca, Roche, Bristol Myers Squibb, Exelixis, Ipsen Merck, MSD, Novrtis, Pfizer, Seattle Genetics, Merck Serono, Astellas Pharma, Johnson & Johnson, Eisai. J. Bedke: Financial Interests, Institutional, Advisory Role: MSD, BMS, Pfizer; Financial Interests, Personal, Advisory Role: AstraZeneca, BMS, Eisai, Ipsen, MSD, Novartis, Roche, EUSA Pharma, Nektar, Pfizer; Financial Interests, Institutional, Research Grant: Eisai, Ipsen, MSD, Novartis, Roche, Pfizer. T.S. Waddell: Financial Interests, Personal, Advisory Role: Roche, Pfizer, Ipsen, Eisai, Bristol Myers Squibb Merck Sharp & Dohme; Financial Interests, Personal, Other, Travel Expenses: EUSA Pharma, Bristol Myers Squibb, Ipsen; Financial Interests, Personal, Officer, Honoraria: Pfizer, Ipsen, Bristol Myers Squibb, EUSA Pharma; Financial Interests, Personal, Research Grant: Bristol Myers Squibb, Pfizer, Ipsen, Merck Sharp & Dohme, Roche, Eisai. D. Nosov: Financial Interests, Personal, Advisory Role: Bayer, Pfizer. F. Pouliot: Financial Interests, Personal, Other, Honoraria: Tersera, Janssen, Astellas, Bayer, Sanofi, Ferring; Financial Interests, Personal, Advisory Role: Tersera, Janssen, Astellas, Bayer, Sanofi, Ferring, Merck; Financial Interests, Personal, Other, Travel Expenses: Amgen; Financial Interests, Personal, Speaker’s Bureau: Janssen; Financial Interests, Institutional, Research Grant: Astellas, Bayer. D. Soulieres: Financial Interests, Personal, Other, Honoraria: Merck, BMS, Pfizer; Financial Interests, Personal, Advisory Role: Merck; Financial Interests, Institutional, Research Grant: Merck, BMS, Pfizer. B. Melichar: Financial Interests, Personal, Other, Honoraria: Roche, Pfizer, BMS, Astellas, Novartis, Bayer, MSD, Merck Serono, Sanofi, Servier, AstraZeneca, Amgen, Janssen, Eisai, E. Lilly, Pierre Farbre; Financial Interests, Personal, Advisory Role: Roche, Pfizer, BMS, Astellas, Novartis, Bayer, MSD, Merck Serono, Sanofi, Servier, AstraZeneca, Amgen, Janssen, Eisai, E. Lilly, Pierre Farbre; Financial Interests, Personal, Other, Travel Expenses: Merck Serono, BMS. S. Azevedo: Financial Interests, Institutional, Research Grant: Merck. R.S. McDermott: Financial Interests, Personal, Advisory Board: Amgen, Bayer, BMS, Clovis, Janssen, Pfizer; Financial Interests, Personal, Invited Speaker: Astellas, Ipsen, MSD; Financial Interests, Institutional, Principal Investigator: Astellas, Bayer, BMS, Clovis, MSD, Regeneron. D. Borchiellini: Financial Interests, Personal, Advisory Role: Astellas, AstraZeneca, BMS, Ipsen, Janseen, Merck Serrono, MSD, Pfizer, Sanofi; Financial Interests, Personal, Other, Travel Expenses: BMS, Ipsen, Janssen, Pfizer; Financial Interests, Institutional, Research Grant: Astellas, AstraZeneca, BMS, Exelixis, Infinity, Janssen, MSD, Pfizer, Roche. J. Lin: Financial Interests, Personal, Full or part-time Employment: Merck & Co., Inc.; Financial Interests, Personal, Stocks/Shares: Merck & Co., Inc. J. Burgents: Financial Interests, Personal, Full or part-time Employment: Merck & Co., Inc.; Financial Interests, Personal, Stocks/Shares: Merck & Co., Inc. L.R. Molife: Financial Interests, Personal, Stocks/Shares: MSD; Financial Interests, Personal, Full or part-time Employment: MSD. E.R. Plimack: Financial Interests, Personal, Advisory Board: BMS,Calithera, Genentech, Janssen, MEI Pharma, Merck, Pfizer, Seattle Genetics, AstraZeneca, Infinity Pharma,; Financial Interests, Institutional, Principal Investigator: Astellas, BMS, Genentech, Merck; Non-Financial Interests, Personal, Other, Board of Directors: ASCO. B. Rini: Financial Interests, Personal, Advisory Role: BMS, Pfizer, GNE/Roche, Aveo, Synthorx, Compugen, Merck, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, GSK, Shionogi, Eisai; Financial Interests, Personal, Leadership Role: SITC; Financial Interests, Personal, Stocks/Shares: PTC Therapeutics; Financial Interests, Personal, Other, Travel expenses: Merck, Pfizer, BMS, Aveo; Financial Interests, Institutional, Research Grant: Pfizer, Hoffman-LaRoche, Incyte, AstraZeneca, Taris, Seattle Genetics, Arrowhead Pharmaceuticals, Immunomedics, BMS, Mirati Therapeutics, Merck, Surface Oncology, Dragonfly Therapeutics, Aravive, Exelixis. All other authors have declared no conflicts of interest.