By Dr. Idir Ouzaid
Localised Disease
After more than 30 years of failed attempts, an immuno-oncology (IO) agent has finally showed a substantial benefit in localised high-risk renal cell carcinoma after nephrectomy. Recurrence after a complete resection of high-risk disease remains an unmet need in the management of kidney cancer. In fact, half of patients will experience a disease progression with potentially fatal evolution.
KEYNOTE-564 is a phase-III multicentre trial in this setting that was designed to assess pembrolizumab vs placebo in patients with histologically confirmed clear cell renal cell carcinoma (ccRCC) with intermediate-high risk (pT2, Gr 4 or sarcomatoid, N0 M0; or pT3, any Gr, N0 M0), high-risk (pT4, any Gr, N0 M0; or pT any stage, any Gr, N+ M0), or M1 NED (no evidence of disease after the primary tumour + soft tissue metastases were completely resected ≤1 year from nephrectomy) according to the Leibovich risk classification (Abstract #LBA5, watch interview with Prof. Necchi on this study). In this trial, patients received one year of pembrolizumab (200 mg Q3W). The primary endpoint was disease-free survival (DFS). Overall survival (OS) was a key secondary endpoint as well as safety.
Overall, 994 patients were randomised 1:1 to pembrolizumab (n=496) or placebo (n=498). As for the data cut-off date, median follow-up was 24.1 (14.9−41.5) mo. The primary endpoint of DFS was met (median not reached in both arms, HR 0.68, 95% CI 0.53−0.87; P=0.0010). The estimated DFS rate at 24 mo was 77.3% with pembrolizumab vs 68.1% with placebo. A total of 51 OS events was observed (18 in the pembrolizumab arm, 33 in the placebo arm). Median OS was not reached in both arms (HR 0.54, 95% CI 0.30−0.96; P=0.0164 [one-sided]); the p-value did not cross the statistical hypothesis testing boundary. The estimated OS rate at 24 mo was 96.6% with pembrolizumab vs 93.5% with placebo. 470 patients (96.3%) and 452 patients (91.1%) experienced ≥1 all-cause adverse events (AEs) with pembrolizumab vs placebo, respectively. Grade 3-5 all-cause AEs occurred in 158 patients (32.4%) with pembrolizumab and 88 patients (17.7%) with placebo. No deaths related to pembrolizumab occurred.
It is noteworthy that 10% of the patients discontinued treatment because of AEs after a median of seven cycles after nephrectomy. Unlike adjuvant Tyrosine Kinase Inhibitors (TKI), which showed an inconsistent benefit in major AEs, the safety profile of adjuvant pembrolizumab seemed appealing. However, mature OS data are needed before including adjuvant IO in the clinical practice.
Metastatic Disease
An update on the Keynote-426 study was presented at ASCO 2021 (Abstract #4500), too, after 42 months of follow up. The Keynote-426 is a phase-III trial that assesses the use of pembrolizumab plus axitinib versus sunitinib as a first-line therapy for advanced ccRCC. The first and extended analysis showed a consistent benefit of pembrolizumab + axitinib over sunitinib. After a median follow-up of 42.8 mo (range 35.6-50.6), 418 patients had died: 193 (44.7%) of 432 patients in the pembrolizumab + axitinib arm vs 225 (52.4%) of 429 patients in the sunitinib arm. As previous reports had indicated as well, pembrolizumab + axitinib improved OS (median: 45.7 vs 40.1 mo; HR, 0.73 [95% CI, 0.60-0.88]; P<0.001) and PFS (median: 15.7 vs 11.1 mo; HR, 0.68 [95% CI, 0.58-0.80]; P<0.0001) compared with sunitinib. The 42-mo OS rate was 57.5% with pembrolizumab + axitinib vs 48.5% with sunitinib; the 42-mo PFS rate was 25.1% with pembrolizumab + axitinib vs 10.6% with sunitinib. The safety profile was of no specific concern after this analysis, just like previously reported. Of all trials examining possible PD–1/L1-immunotherapy-and-VEGF/VEGFR-inhibitor combinations as a first-line treatment for RCC, Keynote-426 is the most mature study that shows the efficacy of such approach over TKI-only therapy.
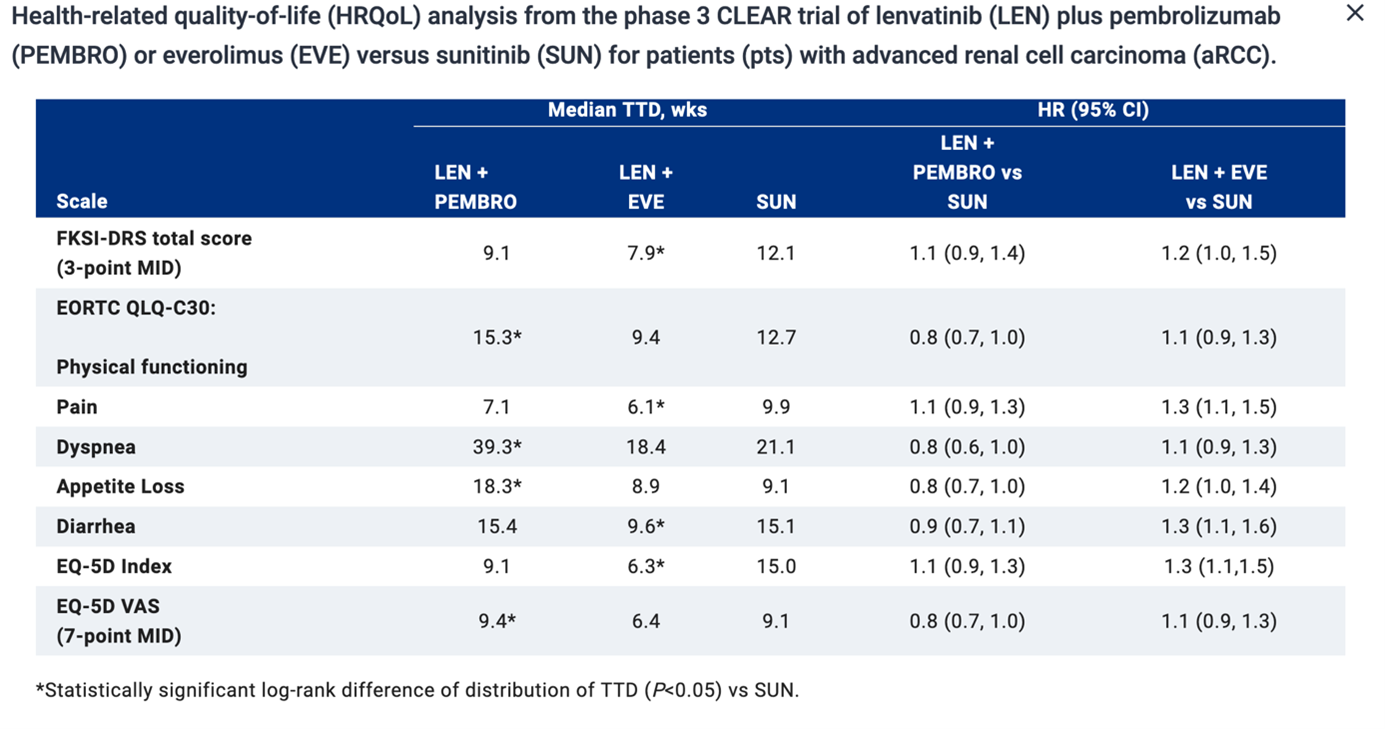
Health-related quality-of-life (HRQoL) outcomes for patients treated for metastatic renal cell carcinoma is of the utmost importance to a patient, drug approval agencies, patient advocacy groups, and health care professionals. Therefore, the analysis of the phase-III CLEAR trial of lenvatinib plus pembrolizumab or everolimus versus sunitinib for patients with advanced renal cell carcinoma was presented during the meeting as well (Abstract #4502). In this trial, pembrolizumab (200 mg Q3W) + Lenvatinib (20 mg QD) demonstrated a superiority over sunitinib in terms of PFS, OS, ORR. HRQoL was assessed per FKSI-DRS, EORTC QLQ-C30, and EuroQoL EQ-5D-3L, at baseline on day 1 of the subsequent three-week cycles starting with cycle 2 and at the off-treatment visit. Compared with SUN, patients in the LEN + PEMBRO group had similar or better symptoms and HRQoL (see table 1). The clinical benefit of pembrolizumab +Lenvatinib is not offset by increased toxicity.
References
1. Choueiri TK et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for patients with renal cell carcinoma: Randomized, double-blind, phase III KEYNOTE-564 study. J Clin Oncol 39, 2021 (suppl 18; LBA5)
2. Rini BI et al.Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): Results from 42-month follow-up of KEYNOTE-426. J Clin Oncol 39, 2021 (suppl 15; abstr 4500)
3. Motzer RJ et al. Health-related quality-of-life (HRQoL) analysis from the phase 3 CLEAR trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) or everolimus (EVE) versus sunitinib (SUN) for patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol 39, 2021 (suppl 15; abstr 4502)