Background
Cytoreductive nephrectomy (neph) remains controversial in the management of patients (pts) with mRCC; the role of prior neph in pts treated with immune checkpoint inhibitor remains unknown. In this extended follow-up analysis of the phase III JAVELIN Renal 101 trial (NCT02684006), we assessed the role of prior neph in pts with mRCC presenting with synchronous metastases at the time of diagnosis and treated with A + Ax or S.
Methods
Efficacy outcomes were assessed from the third interim analysis in pts with mRCC who presented with M1 disease at the time of diagnosis and had undergone prior neph or no neph in the A + Ax and S arms. Multivariate Cox regression analyses were used to assess hazard ratios for overall survival (OS) and progression-free survival (PFS; investigator assessment per RECIST 1.1). Logistic regression method was used to obtain the odds ratio for objective response (investigator assessment per RECIST 1.1).
Results
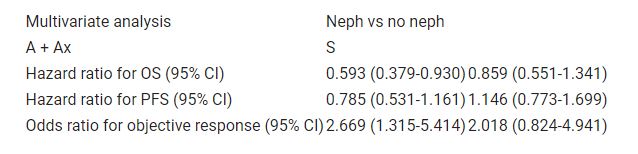
412 of 886 pts in JAVELIN Renal 101 presented with M1 disease at diagnosis. Of these pts, 126 (A + Ax) and 147 (S) had undergone prior neph and 72 (A + Ax) and 67 (S) had no neph. Compared with the neph group, a higher proportion of pts in the no-neph group were older (42% vs 32% were ≥65 years), had impaired PS (ECOG PS 1, 54% vs 40%), and had poor prognosis (40% vs 16% by IMDC criteria); fewer pts had PD-L1+ tumors (40% vs 77%). To assess the impact of neph, these parameters were adjusted in the multivariate model. Post hoc analyses showed that in the A + Ax arm, OS and confirmed objective response were significantly improved in pts with neph vs no neph; however, no differences were observed in the S arm (Table). PFS was numerically longer in pts with neph vs no neph in the A + Ax arm but not in the S arm.
Conclusions
Pts who presented with M1 disease at the time of diagnosis and had undergone prior neph had superior efficacy outcomes vs no neph with A + Ax but not with S. Table: 665P
Clinical trial identification
NCT02684006.
Editorial acknowledgement
Medical writing support was provided by Kakoli Parai of ClinicalThinking and funded by Pfizer and Merck KGaA, Darmstadt, Germany.
Legal entity responsible for the study
Pfizer and Merck KGaA, Darmstadt, Germany.
Funding
Pfizer and Merck KGaA, Darmstadt, Germany.
Disclosure
M. Grimm: Financial Interests, Personal, Other, Honoraria: Astellas Pharma; Financial Interests, Personal, Other, Honoraria: AstraZeneca; Financial Interests, Personal, Other, Honoraria: Bristol-Myers Squibb; Financial Interests, Personal, Other, Honoraria: Merck Sharp & Dohme; Financial Interests, Personal, Other, Honoraria: Pfizer; Financial Interests, Personal, Other, Honoraria: Ipsen; Financial Interests, Personal, Other, Honoraria: Merck Serono; Financial Interests, Personal, Other, Honoraria: EUSA Pharma; Financial Interests, Personal, Advisory Role: AstraZeneca; Financial Interests, Personal, Advisory Role: Bristol-Myers Squibb; Financial Interests, Personal, Advisory Role: Ipsen; Financial Interests, Personal, Advisory Role: Merck Sharp & Dohme; Financial Interests, Personal, Advisory Role: Pfizer; Financial Interests, Personal, Advisory Role: Astellas Pharma; Financial Interests, Personal, Advisory Role: EUSA Pharma; Financial Interests, Personal, Advisory Role: Merck Serono; Financial Interests, Personal, Advisory Role: Roche Pharma AG; Financial Interests, Personal, Advisory Role: Takeda; Financial Interests, Personal, Advisory Role: Eisai; Financial Interests, Institutional, Research Grant: Bristol-Myers Squibb; Financial Interests, Institutional, Research Grant: Intuitive Surgical; Financial Interests, Personal, Other, Travel, Accommodations, Expenses: Bristol-Myers Squibb; Financial Interests, Personal, Other, Travel, Accommodations, Expenses: Merck Serono. M. Oya: Financial Interests, Personal, Invited Speaker: Pfizer; Financial Interests, Personal, Invited Speaker: Novartis; Financial Interests, Personal, Invited Speaker: Bayer; Financial Interests, Personal, Invited Speaker: Ono; Financial Interests, Personal, Invited Speaker: Bristol-Myers Squibb; Financial Interests, Personal, Invited Speaker: Takeda; Financial Interests, Personal, Invited Speaker: Merck Sharp & Dohme. T.K. Choueiri: Financial Interests, Personal, Stocks/Shares: Pionyr; Financial Interests, Personal, Stocks/Shares: Tempest Therapeutics; Financial Interests, Personal, Other, Honoraria: Alexion Pharmaceuticals; Financial Interests, Personal, Other, Honoraria: Alligent; Financial Interests, Personal, Other, Honoraria: Analysis Group; Financial Interests, Personal, Other, Honoraria: ASCO; Financial Interests, Personal, Other, Honoraria: AstraZeneca; Financial Interests, Personal, Other, Honoraria: Bayer; Financial Interests, Personal, Other, Honoraria: Bristol-Myers Squibb; Financial Interests, Personal, Other, Honoraria: Cerulean Pharma; Financial Interests, Personal, Other, Honoraria: Clinical Care Options; Financial Interests, Personal, Other, Honoraria: Corvus Pharmaceuticals; Financial Interests, Personal, Other, Honoraria: Eisai; Financial Interests, Personal, Other, Honoraria: EMD Serono; Financial Interests, Personal, Other, Honoraria: Exelixis; Financial Interests, Personal, Other, Honoraria: Foundation Medicine; Financial Interests, Personal, Other, Honoraria: Genetech/Roche; Financial Interests, Personal, Other, Honoraria: GlaxoSmithKline; Financial Interests, Personal, Other, Honoraria: Harborside Press; Financial Interests, Personal, Other, Honoraria: Heron; Financial Interests, Personal, Other, Honoraria: Ipsen; Financial Interests, Personal, Other, Honoraria: Kidney Cancer Association; Financial Interests, Personal, Other, Honoraria: Lancet Oncology; Financial Interests, Personal, Other, Honoraria: Lilly; Financial Interests, Personal, Other, Honoraria: Lpath; Financial Interests, Personal, Other, Honoraria: Merck Sharp & Dohme; Financial Interests, Personal, Other, Honoraria: Michael J. Hennessy Associates; Financial Interests, Personal, Other, Honoraria: Navinata Health; Financial Interests, Personal, Other, Honoraria: NCCN; Financial Interests, Personal, Other, Honoraria: Novartis; Financial Interests, Personal, Other, Honoraria: Peloton Therapeutics; Financial Interests, Personal, Other, Honoraria: Pfizer; Financial Interests, Personal, Other, Honoraria: PlatformQ Health; Financial Interests, Personal, Other, Honoraria: Prometheus; Financial Interests, Personal, Other, Honoraria: Sanofi/Aventis; Financial Interests, Personal, Other, Honoraria: New England Journal of Medicine; Financial Interests, Personal, Advisory Role: Alexion Pharmaceuticals; Financial Interests, Personal, Advisory Role: Alligent; Financial Interests, Personal, Advisory Role: Analysis Group; Financial Interests, Personal, Advisory Role: ASCO; Financial Interests, Personal, Advisory Role: AstraZeneca; Financial Interests, Personal, Advisory Role: Bayer; Financial Interests, Personal, Advisory Role: Bristol-Myers Squibb; Financial Interests, Personal, Advisory Role: Cerulean Pharma; Financial Interests, Personal, Advisory Role: Clinical Care Options; Financial Interests, Personal, Advisory Role: Corvus Pharmaceuticals; Financial Interests, Personal, Advisory Role: Eisai; Financial Interests, Personal, Advisory Role: EMD Serono; Financial Interests, Personal, Advisory Role: ESMO; Financial Interests, Personal, Advisory Role: Exelixis; Financial Interests, Personal, Advisory Role: Foundation Medicine; Financial Interests, Personal, Advisory Role: GlaxoSmithKline; Financial Interests, Personal, Advisory Role: Harborside Press; Financial Interests, Personal, Advisory Role: Heron; Financial Interests, Personal, Advisory Role: Ipsen; Financial Interests, Personal, Advisory Role: Kidney Cancer Association; Financial Interests, Personal, Advisory Role: Lancet Oncology; Financial Interests, Personal, Advisory Role: Lilly; Financial Interests, Personal, Advisory Role: Lpath; Financial Interests, Personal, Advisory Role: Merck Sharp & Dohme; Financial Interests, Personal, Advisory Role: Michael J. Hennessy Associates; Financial Interests, Personal, Advisory Role: Navinata Health; Financial Interests, Personal, Advisory Role: NCCN; Financial Interests, Personal, Advisory Role: Novartis; Financial Interests, Personal, Advisory Role: Peloton Therapeutics; Financial Interests, Personal, Leadership Role: Pfizer; Financial Interests, Personal, Leadership Role: PlatformQ Health; Financial Interests, Personal, Leadership Role: Prometheus; Financial Interests, Personal, Advisory Role: Roche/Genentech; Financial Interests, Personal, Advisory Role: Sanofi/Aventis; Financial Interests, Personal, Advisory Role: New England Journal of Medicine; Financial Interests, Institutional, Research Grant: Agensys; Financial Interests, Institutional, Research Grant: Analysis Group; Financial Interests, Institutional, Research Grant: AstraZeneca; Financial Interests, Institutional, Research Grant: Bayer; Financial Interests, Institutional, Research Grant: Bristol-Myers Squibb; Financial Interests, Institutional, Research Grant: Calithera Biosciences; Financial Interests, Institutional, Research Grant: Celldex; Financial Interests, Institutional, Research Grant: Cerulean Pharma; Financial Interests, Institutional, Research Grant: Congressionally Directed Medical Research Programs (DOD); Financial Interests, Institutional, Research Grant: Corvus Pharmaceuticals; Financial Interests, Institutional, Research Grant: Eisai; Financial Interests, Institutional, Research Grant: Exelixis; Financial Interests, Institutional, Research Grant: Foundation Medicine; Financial Interests, Institutional, Research Grant: Gateway for Cancer Research; Financial Interests, Institutional, Research Grant: GlaxoSmithKline; Financial Interests, Institutional, Research Grant: Ipsen; Financial Interests, Institutional, Research Grant: Merck Sharp & Dohme; Financial Interests, Institutional, Research Grant: NCI; Financial Interests, Institutional, Research Grant: Novartis; Financial Interests, Institutional, Research Grant: Peloton Therapeutics; Financial Interests, Institutional, Research Grant: Pfizer; Financial Interests, Institutional, Research Grant: Prometheus; Financial Interests, Institutional, Research Grant: Roche; Financial Interests, Institutional, Research Grant: Roche/Genentech; Financial Interests, Institutional, Research Grant: Seattle Genetics/Astellas; Financial Interests, Institutional, Research Grant: Takeda; Financial Interests, Institutional, Research Grant: Tracon Pharmaceuticals; Financial Interests, Personal, Other, owns patents, royalties, or other intellectual property: PCT/US2018/058430, PCT/US2018/12209; Financial Interests, Personal, Other, Honoraria: UpToDate. M. Schmidinger: Financial Interests, Personal, Advisory Role: Roche; Financial Interests, Personal, Advisory Role: Bristol-Myers Squibb; Financial Interests, Personal, Advisory Role: Merck KGaA, Darmstadt, Germany; Financial Interests, Personal, Advisory Role: Merck Sharp & Dohme; Financial Interests, Personal, Advisory Role: Pfizer; Financial Interests, Personal, Advisory Role: EUSA; Financial Interests, Personal, Advisory Role: Eisai; Financial Interests, Personal, Advisory Role: Ipsen; Financial Interests, Personal, Advisory Role: Exelixis; Financial Interests, Personal, Advisory Role: Alkermes plc; Financial Interests, Personal, Other, Honoraria for Lectures: Bristol-Myers Squibb; Financial Interests, Personal, Other, Honoraria for Lectures: Merck KGaA, Darmstadt, Germany; Financial Interests, Personal, Other, Honoraria for Lectures: Merck Sharp & Dohme; Financial Interests, Personal, Other, Honoraria for Lectures: Pfizer; Financial Interests, Personal, Other, Honoraria for Lectures: EUSA; Financial Interests, Personal, Other, Honoraria for Lectures: Eisai; Financial Interests, Personal, Other, Honoraria for Lectures: Ipsen; Financial Interests, Personal, Other, Honoraria for Lectures: Exelixis. D.I. Quinn: Financial Interests, Personal, Invited Speaker, Educational lectures: Astellas; Financial Interests, Personal, Advisory Board, Advisory Board: AstraZeneca; Financial Interests, Personal, Advisory Board, Advisory Board: Bayer; Financial Interests, Personal, Advisory Board, Advisory Board: BMS; Financial Interests, Personal, Advisory Board, Advisory Board: Genentech; Financial Interests, Personal, Advisory Board, Advisory Board: Roche; Financial Interests, Personal, Advisory Board, Advisory Board: Merck; Financial Interests, Personal, Advisory Board, Advisory Board: Exelixis; Financial Interests, Personal, Advisory Board, Advisory Board: EMD Serono; Financial Interests, Personal, Advisory Board, Advisory Board: Seattle Genetics; Financial Interests, Personal, Invited Speaker, Educational lectures and Advisory Board: Pfizer; Financial Interests, Personal, Other, Data Safety Monitoring: Eisai; Financial Interests, Institutional, Invited Speaker, phase 2 study, phase 3 study: Pfizer; Financial Interests, Institutional, Funding, phase 2 study: Merck; Financial Interests, Institutional, Funding, phase 2 trial: Genentech; Financial Interests, Personal and Institutional, Invited Speaker, Coordination of international phase 2/3 trial: Bayer. G. Gravis Mescam: Financial Interests, Personal, Other, Travel, Accommodations, Expenses: Janssen Oncology; Financial Interests, Personal, Other, Travel, Accommodations, Expenses: Bristol-Myers Squibb; Financial Interests, Personal, Other, Travel, Accommodations, Expenses: Astellas Pharma; Financial Interests, Personal, Other, Travel, Accommodations, Expenses: Pfizer; Financial Interests, Personal, Other, Travel, Accommodations, Expenses: Ipsen; Financial Interests, Personal, Other, Travel, Accommodations, Expenses: Sanofi. E. Verzoni: Financial Interests, Personal, Advisory Role: Pfizer; Financial Interests, Personal, Advisory Role: MSD; Financial Interests, Personal, Advisory Role: Merck KGaA; Financial Interests, Personal, Advisory Role: Ipsen. A.J.M. Van den Eertwegh: Financial Interests, Institutional, Research Grant: Roche; Financial Interests, Institutional, Research Grant: Sanofi; Financial Interests, Institutional, Research Grant: TEVA; Financial Interests, Personal and Institutional, Advisory Role: Bristol-Myers Squibb; Financial Interests, Personal and Institutional, Advisory Role: Merck Sharp & Dohme; Financial Interests, Personal and Institutional, Advisory Role: AMGEN; Financial Interests, Personal and Institutional, Advisory Role: Pfizer; Financial Interests, Personal and Institutional, Advisory Role: Merck & Co.; Financial Interests, Personal and Institutional, Advisory Role: Novartis; Financial Interests, Personal and Institutional, Advisory Role: Ipsen; Financial Interests, Personal and Institutional, Advisory Role: Pierre Fabre; Financial Interests, Personal and Institutional, Advisory Role: Eisai; Financial Interests, Personal, Speaker’s Bureau: Bristol-Myers Squibb. A. di Pietro: Financial Interests, Personal, Full or part-time Employment: Pfizer; Financial Interests, Personal, Stocks/Shares: Pfizer. M. Mariani: Financial Interests, Personal, Full or part-time Employment: Pfizer; Financial Interests, Personal, Stocks/Shares: Pfizer. J. Wang: Financial Interests, Personal, Full or part-time Employment: Pfizer. D. Thomaidou: Financial Interests, Personal, Full or part-time Employment: Pfizer; Financial Interests, Personal, Full or part-time Employment: Pfizer. L. Albiges: Financial Interests, Institutional, Research Grant: Bristol-Myers Squibb; Financial Interests, Institutional, Advisory Role: Astellas; Financial Interests, Institutional, Advisory Role: AstraZeneca; Financial Interests, Institutional, Advisory Role: Bellerophon; Financial Interests, Institutional, Advisory Role: Bristol-Myers Squibb; Financial Interests, Institutional, Advisory Role: Corvus Pharmaceuticals; Financial Interests, Institutional, Advisory Role: Ipsen; Financial Interests, Institutional, Advisory Role: Janssen; Financial Interests, Institutional, Advisory Role: Merck & Co.; Financial Interests, Institutional, Advisory Role: Merck Sharp & Dohme; Financial Interests, Institutional, Advisory Role: Novartis; Financial Interests, Institutional, Advisory Role: Pfizer; Financial Interests, Institutional, Advisory Role: Springer Healthcare. All other authors have declared no conflicts of interest.