By Dr. Idir Ouzaid
The UROONCO RCC editorial board is pleased to share with you the top 5 renal cell carcinoma highlights from the recently-concluded ASCO20 Virtual meeting.
1. KEYNOTE-426: Pembrolizumab plus Axitinib versus Sunitinib as First-Line Therapy for Advanced Renal Cell Carcinoma (Absract#5001)
Data of the Keynote 426 was updated with a minimum 23 months (7 months when the study was first released). The study met both primary endpoints of improving overall survival (OS) and PFS when it was presented at the GU ASCO meeting in 2019. The OS at 1 year (90% vs 78%, HR 0.53) and objective response rates was 59% with pembro/axi compared with 36% with sunitinib. The benefit of pembro/axi was observed across all IMDC risk groups, regardless of PD-L1 expression.
This update showed 22% of patients are still on treatment in the pembro/axi arm compared with 17.9% in the sunitinib arm. OS analysis shows that 74% of patients survived from the pembro/axi arm at 24 months, compared with 66% of patients on sunitinib arm. Median survival is not yet reached with pembro/axi and was 35.7 months with sunitinib.
For patients with favourable-risk disease, there was no significant difference in OS or PFS, with a median PFS of 20.8 months with pembro/axi and 18 months with sunitinib. However, for patients with IMDC intermediate or poor-risk disease, there were significant differences in OS and PFS with HR of 0.63 for OS and 0.69 for PFS.
These data presented today continue to show that pembro/axi is superior to sunitinib for most patients with mRCC and landmark analyses show that the depth of tumour shrinkage correlates with OS. The updated 2020 EAU Guidelines introduced this combination therapy in the first-line treatment of metastatic RCC (mRCC) regardless of IMDC risk group.
2. SAVOIR: A Phase III Study of Savolitinib versus Sunitinib in Patients with MET-Driven Papillary Renal Cell Carcinoma (Abstract #5002)
Type 1 papillary RCC is typically associated with MET pathway dysregulation. The MET pathway is associated with tumour growth, angiogenesis, and promotion of metastases, as well as, treatment resistance. MET-driven type papillary RCC was as defined by chromosome 7 copy gain, focal MET or HGF gene amplification, or MET kinase domain mutations.
After promising results in the phase II study, the SAVOIR trial investigated whether savolitinib can improve overall survival over sunitinib in MET-driven papillary RCC. In this study, MET-driven tumours (n= ) were randomized in a 1:1 ratio to either savolitinib 600 mg daily or sunitinib 50 mg daily in 4-week on/2-week off cycle.
Overall, 254 patients were screened and only 60 patients met criteria, most due to lack of MET-driven alterations (n=181). Unfortunately, the trial did not meet its primary endpoint of improving progression-free survival. The median PFS was 7 months with savolitinib compared with 5.6 months with sunitinib, HR 0.71, P=0.313.
3. COSMIC-313 Phase III Study of Cabozantinib in Combination with Nivolumab and Ipilimumab in Patients with Previously Untreated Advanced Renal Cell Carcinoma of Intermediate or Poor-Risk (Abstract#TPS5102)
COSMIC-313 is a randomized, double-blind, controlled phase III study evaluating the efficacy and safety of cabozantinib + nivolumab + ipilimumab vs nivolumab + ipilimumab in previously untreated patients with IMDC intermediate or poor-risk advanced RCC. Eligible patients are randomized 1:1 to receive cabozantinib + nivolumab + ipilimumab or nivolumab + ipilimumab in combination with placebo, stratified by IMDC prognostic score and geographic region. The trial design for COSMIC-313 is as follows:
This trial aims to reinforce TKI and IO therapy in the first-line treatment of intermediate and poor-risk RCC. This is supported by previously reported studies assessing the role of cabozantinib alone (CABOSUN) and Cabozantinib associated with nivolumab (CheckMate 9ER study) versus sunitinib in the first-line setting for advanced RCC.
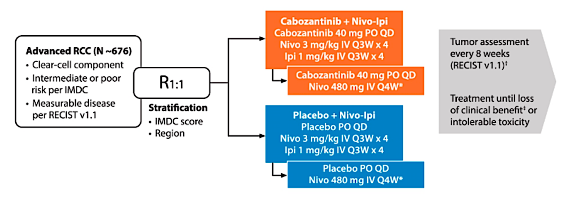
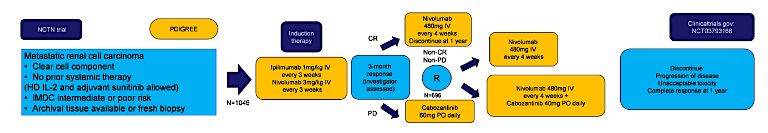
4. PDIGREE: An Adaptive Phase III Trial of PD-Inhibitor Nivolumab and Ipilimumab with VEGF TKI Cabozantinib in Metastatic Untreated Renal Cell Cancer (Abstract#TPS5100)
PDIGREE is a novel adaptive frontline immunotherapy study which upon completion will help answer many important questions regarding treatment duration, when to combine VEGF therapy with IO therapy, and which patients benefit most from IO-VEGF combination upfront versus sequential therapy.
This ongoing study is planning to enrol a total of 1,046 patients. Patients will receive induction therapy per the CheckMate-214 protocol of ipilimumab 1mg/mg + nivolumab 3 mg/kg every 3 weeks for a total of 4 cycles. Following this induction period, the investigator will assess the treatment response to assign the patient into 1 of 3 treatment groups.
For patients with progressive disease, patients will switch to cabozantinib 60 mg daily. For patients with a complete response, they will proceed with maintenance nivolumab. For patients with a partial response or stable disease, patients will be randomly assigned to either maintenance nivolumab or nivolumab in combination with cabozantinib 40 mg daily.
5. Phase II trial of Lenvatinib plus Pembrolizumab for Disease Progression after PD-1/PD-L1 Immune Checkpoint Inhibitor in Metastatic Clear Cell Renal Cell Carcinoma (Abstract#5008)
The frontline standard of care for patients with mRCC involves either IO+IO or IO+TKI combinations. For patients who progress on front-line IO, there is limited data on the efficacy of utilizing combination TKI/immunotherapy in the second line. This study provides data on the use of combination lenvatinib plus pembrolizumab for patients who had progressed on immune checkpoint inhibition.
The study included patients who had at least 2 doses of anti-PD1/PDL1 therapy and patients were given lenvatinib 20 mg daily with pembrolizumab 200 mg every 3 weeks. The primary objective was ORR at 24 weeks.
A total of 104 patients were enrolled and 69% of patients were still receiving treatment at the time of data cut-off. In terms of the baseline characteristics, 75% of patients had prior nephrectomy, 36% of patients had favourable-risk disease, and 42% were classified as PD-L1 positive defined as a CPS≥1.
The objective response of Len/Pembro was 51%, with a median PFS of 11.7 months and median duration of response of 12 months. Only 5% of patients had progressive disease. The progression-free survival was 11.7 months with 45% without progression at 12 months. The OS was 77% at 12 months. The patients had good objective responses regardless of priory therapy. Overall, 43% of patients required a dose reduction and 12% of patients discontinued therapy due to toxicity.