By Dr. Ouzaid
Because of the Covid-19 pandemic, the annual conference of ESMO took place virtually from the 19th to the 21st of September 2020 instead of the planned meeting in Madrid. Some high-quality sessions involved kidney cancer, including the new kid on the block in the front-line treatments of metastatic renal cell carcinoma after the result of the Checkmate 9ER phase-3 trial.
So far, new strategies for systemic treatment of renal cell carcinoma have included nivolumab + Ipilimumab for the intermediate and unfavourable IMDC risk group and Prembrolizumab + Axitinib for all risk groups. Both strategies have respectively shown a survival benefit compared to sunitinib in the Checkmate 214 and Keynote 426 phase-3 trials.
Checkmate 9ER is a phase III, open-label, international randomised controlled trial among patients with previously untreated, histologically confirmed advanced or metastatic renal cell carcinoma with a clear cell component. Patients were randomised in a 1:1 fashion to receive nivolumab 240mg IV q2 weeks plus cabozantinib 40mg PO daily or sunitinib 50mg PO daily for 4 weeks in 6-week cycles. Randomisation was stratified by IMDC risk score, tumour PD-L1 expression, and region. Patients continued with the study therapy until a disease progression or an unacceptable toxicity. The study was powered for progression-free survival as a primary outcome. This was assessed by blinded central review. Secondary outcomes included overall survival, objective response rate, and safety/toxicity.
A total of 651 patients were randomised; 323 to nivolumab plus cabozantinib and 328 to sunitinib. Of the study cohort, 22.6% had favourable-risk disease, 57.6% had intermediate-risk disease, and 19.7% had poor-risk disease. Approximately one-quarter (24.9%) had PD-L1 expression >1%. The main findings are summarised in table 1.
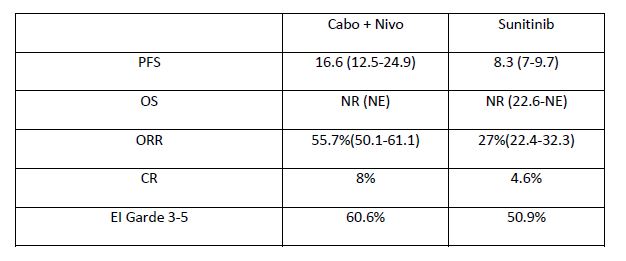
Cabozantinib has also been assessed in combination with atezolizumab in the first-line treatment of metastatic RCC. The Cosmic 021 study, which was also reported during ESMO 2020, tested an escalation dose of cabozantinib from 40 mg to 60 mg within this combination, and it demonstrated encouraging clinical efficacy with reasonable safety profiles. Findings suggested that PD-L1+ tumours with high CD8+ T cell infiltrates were more likely to respond to therapy. A Phase-3 study (CONTACT-03) is now taking place to confirm the activity of this combination, comparing cabozantinib +/- atezolizumab in mRCC previously treated with immune checkpoint blockade.Dr. Tony Choueiri from the Dana Faber Cancer Centre in Boston was very confident about the findings because “the trial [had] met all efficacy endpoints (PFS, OS, ORR), using both the independent and investigator-assessed method,” he said in an email exchange with Uroonco. The study being positive for both primary and secondary endpoints, this nivolumab and Cabozantinib combination is likely to be included in the next EAU RCC guidelines. After we had asked whether this new combination involved the “me too” approach (as compared to nivolumab + Ipilimumab and Prembrolizumab + Axitinib), Dr. Choueiri insisted on the superiority of this combination over sunitinib with respect to QoL outcomes before concluding that this study has shown that “patients not only live longer they also live better.”
Finally, although multiple combination therapies are available in this line of treatment, treatment strategy has so far been based on IMDC risk groups only. To improve treatment selection, a biomarker-driven phase-2 trial, the Bionikk trial, was set up with nivolumab and Ipilimumab or a VEGFR Tyrosine Kinase Inhibitor in naïve metastatic kidney cancer patients. The trial was based on the biological model that analysed transcriptomic data from frozen clear cell RCC tumours, which revealed four groups of patients (ccrcc1-4) with immune and angiogenic high/low features which could allow for a better identification of responders to either nivolumab, nivolumab plus ipilimumab, or TKI. ccrcc1 “immune-low” and ccrcc4 “immune-high” tumours have been associated with the poorest outcomes, whereas ccrcc2 “angio-high” and ccrcc3 “normal-like” tumours have been associated with the best outcomes. Therefore, ccrcc1 and ccrcc4 patients were randomised to nivolumab versus nivolumab plus ipilimumab, whereas ccrcc2 and ccrcc3 patients were randomised to receive nivolumab plus ipilimumab versus TKI. Survival data is immature, the take home messages included:
This approach did not rely on IMDC criteria but on biology. While the feasibility of such approach outside a clinical trial is questionable, biomarker-based studies are the way to go when clinical criteria are not sufficient to drive treatment strategies and when various approaches are available.